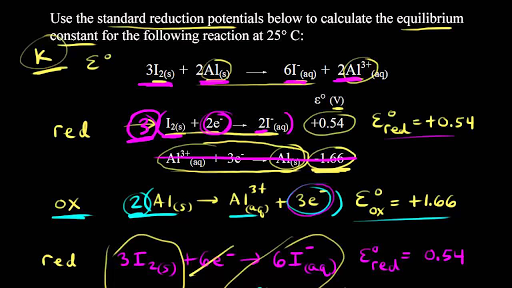
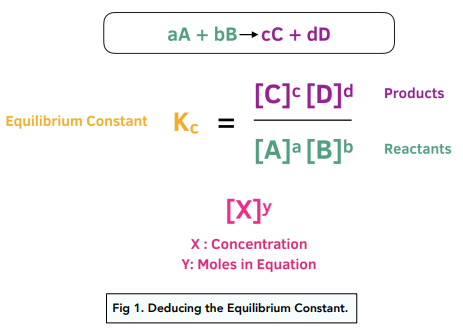
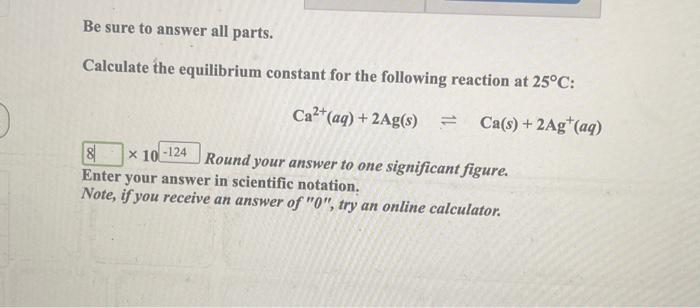
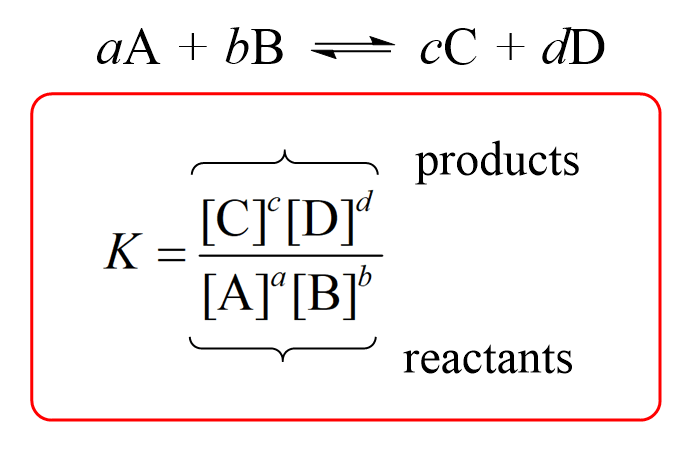
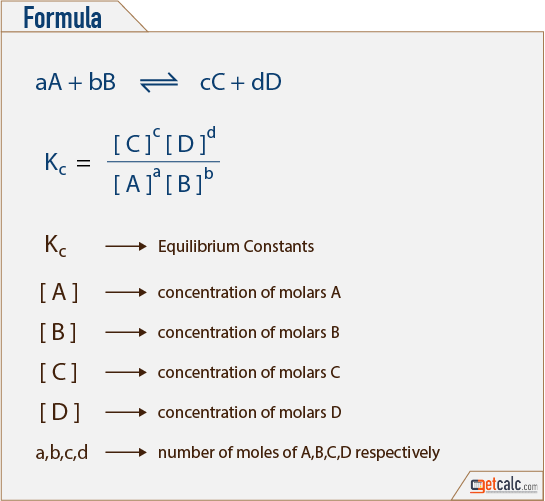
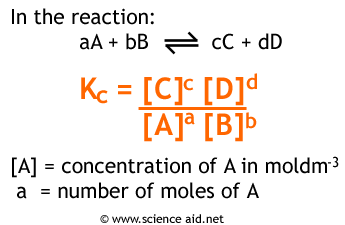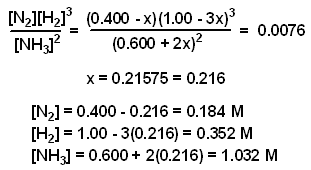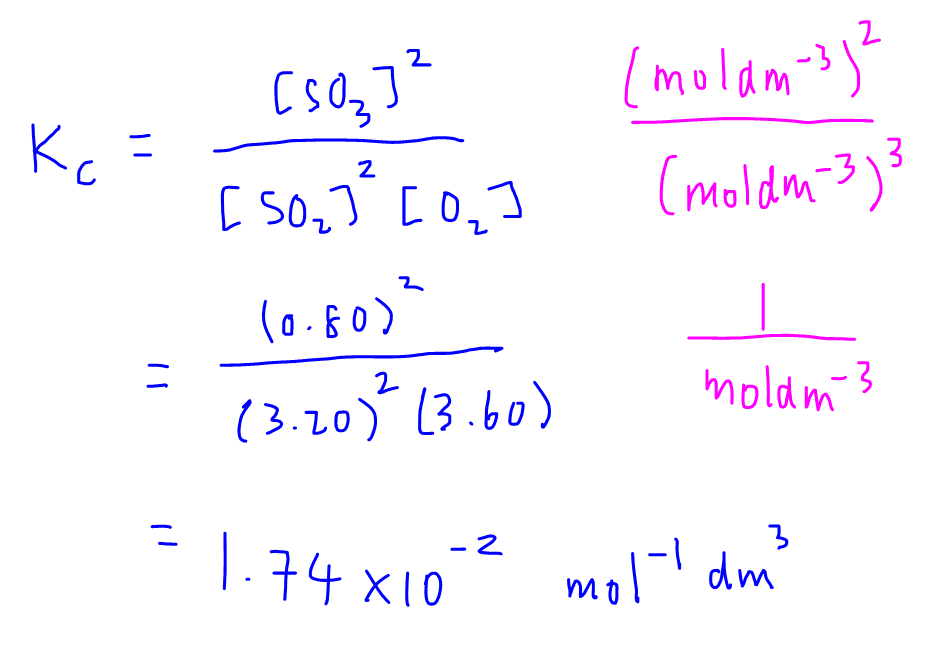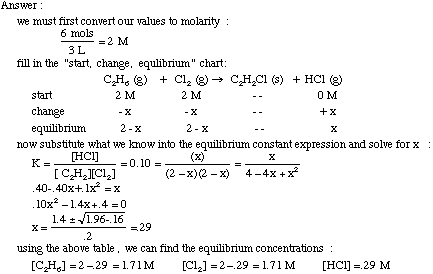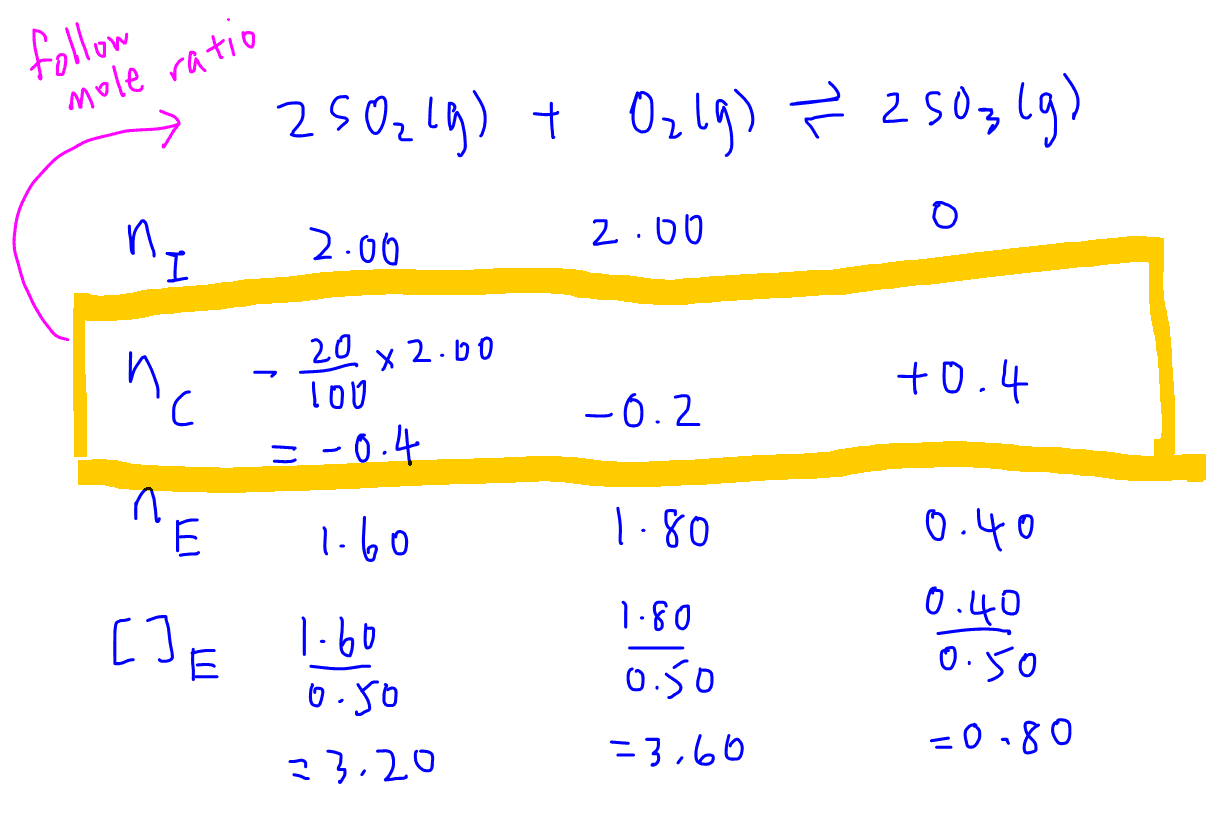Easy tricks to calculate equilibrium constant based problems/Chemical eq... | Simple tricks, Equilibrium, Trick
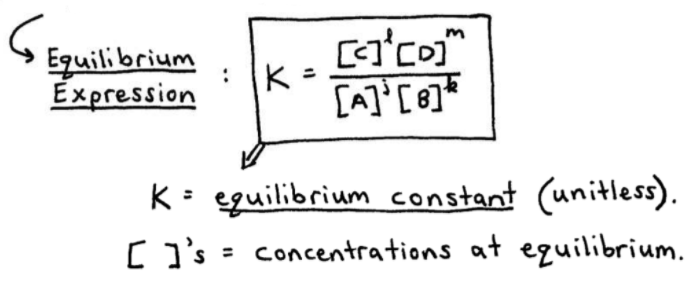
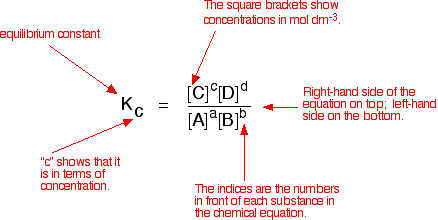
Equilibrium constant has a unit when the number of moles on both sides are not equal, why? If number of moles are not equal then there is no equilibrium then how equilibrium

A Simple Method To Calculate the Temperature Dependence of the Gibbs Energy and Chemical Equilibrium Constants | Journal of Chemical Education
Calculate the equilibrium constant for the reaction at 25∘ C. Fe + CuSO 4⇌ FeSO 4+ CuGiven EO Pi z0=0.44 V ; EO PLu0=0.337 VA. 10+26.33B. 10–20.69C. 10+20.69D. 10–26.33

Calculate the equilibrium constant the reaction, 25^oCCu(s) + 2Ag^+ (aq) → Cu^{+2} (aq) + 2Ag (s)at 25^oC, E^ocell = 0.47 V, R = 8.134 JK^{-1} F = 96500 C is
Calculate the equilibrium constant for the redox reaction at 25°C. Sr(s) + Mg^(2+) → Sr^(2+)(aq) + Mg(s), - Sarthaks eConnect | Largest Online Education Community