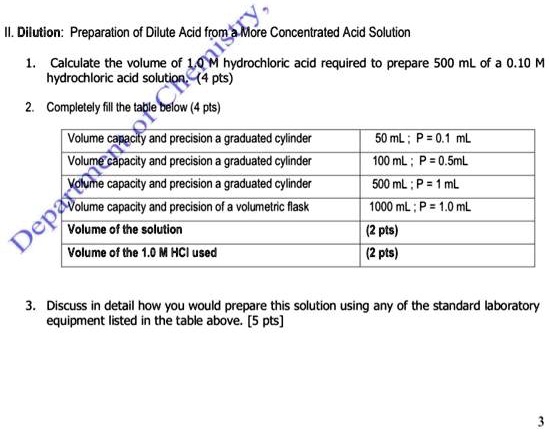
What is the concentration of a solution formed by diluting 190 mL of 18 M HCL solution to 730 mL? | Socratic

SOLVED: Free Energy of Dilution Calculate ΔG for the dilution of aqueous HCl from 87 M to 0.095 M. 45.00 Subani Anetu Tricks 0/5 This discussion is closed.
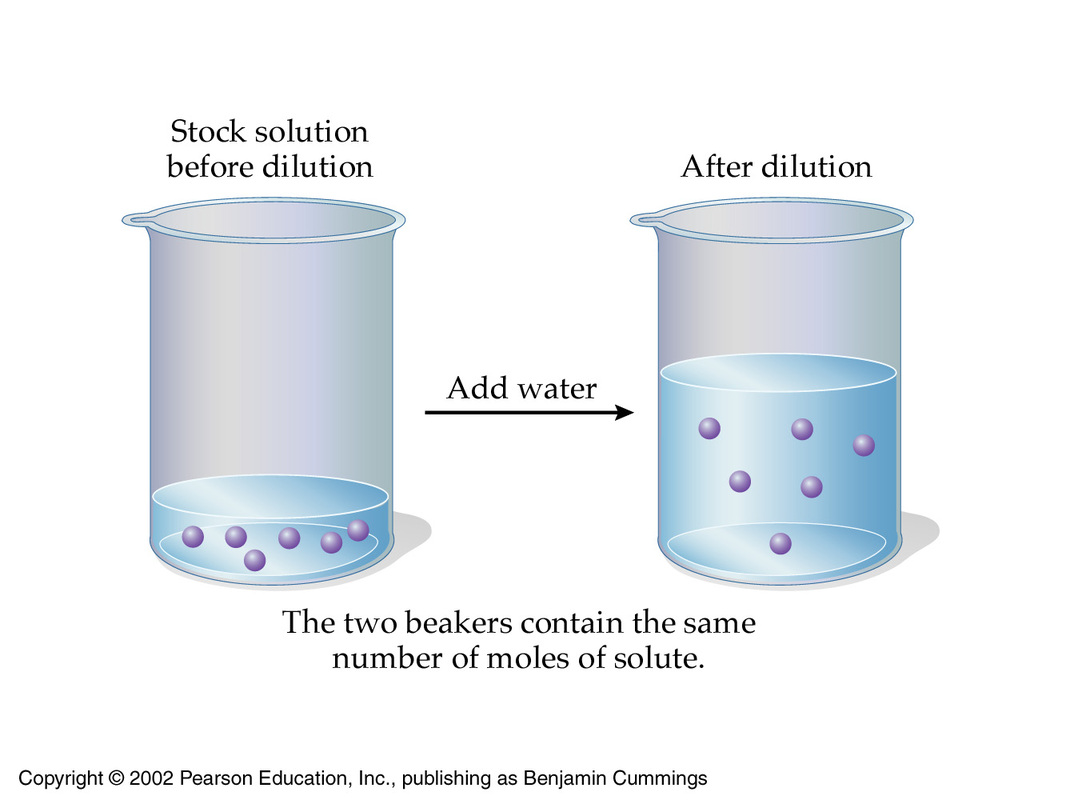
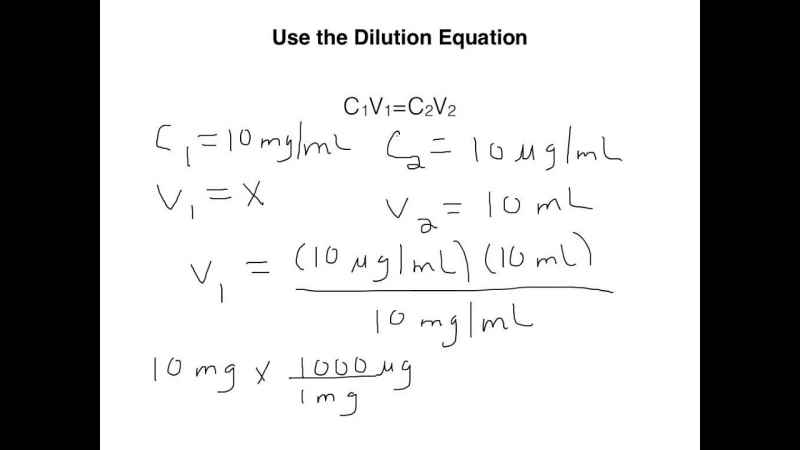
How many milliliters of 12.0 M HCl(aq) must be diluted with water to make exactly 500. mL of 3.00 M hydrochloric acid? | Socratic
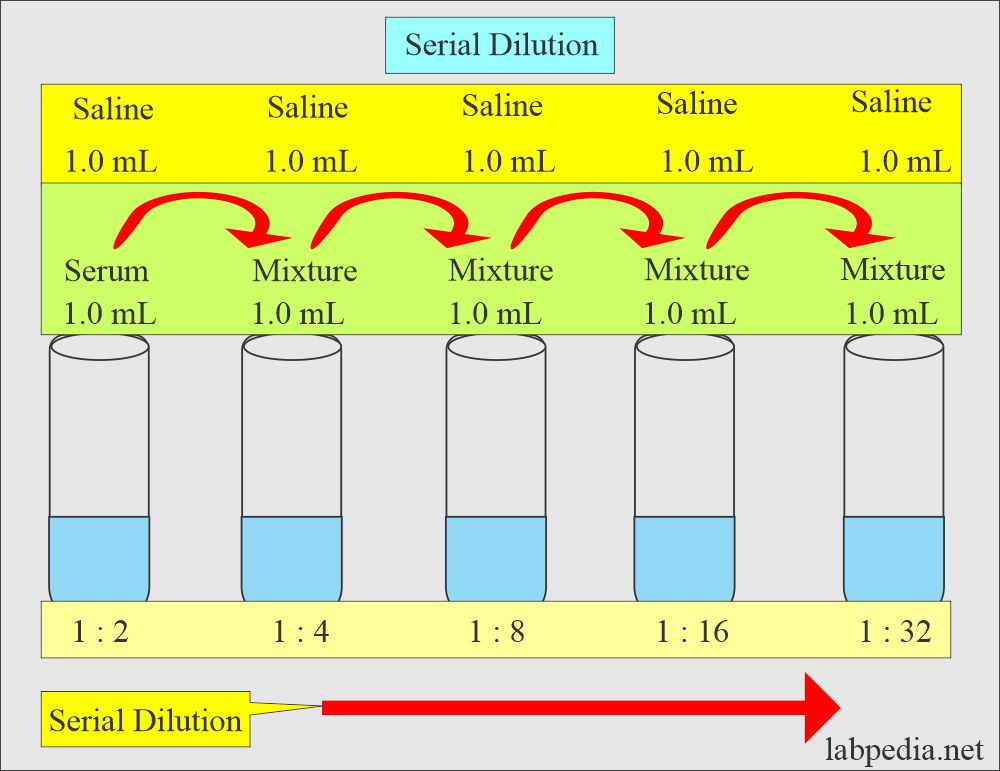
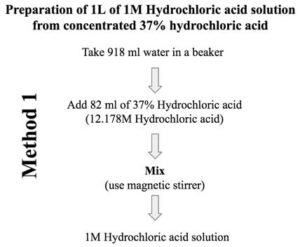
Solutions:- Part 1 - Solutions Preparation used in Clinical Laboratory, and Dilution Formulas - Labpedia.net

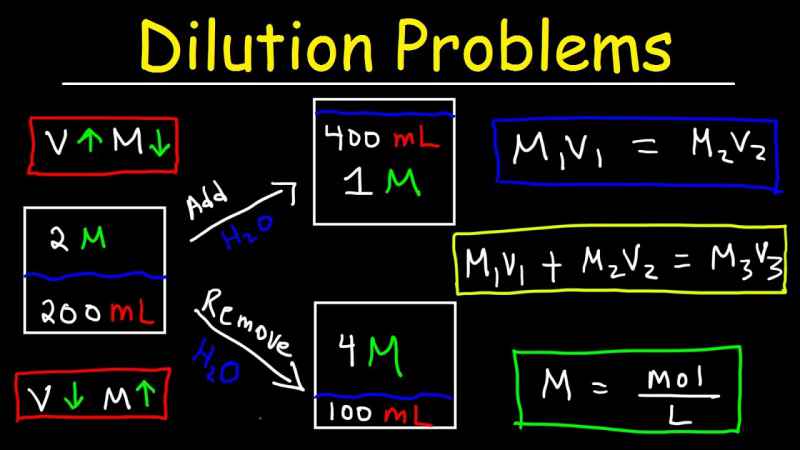
1 Chapter 7 Solutions 7.5 Molarity and Dilution. 2 Molarity (M) Molarity (M) is a concentration term for solutions. gives the moles of solute in 1 L solution. - ppt download
A solution having a pH of 6 is diluted 100 times. Can you calculate the pH of the resulting solution? - Quora